Background: Therapeutic advances in multiple myeloma (MM) have greatly improved the rate and depth of response. Venetoclax (Ven) is a highly selective, potent, oral BCL-2 inhibitor that has synergistic activity with carfilzomib (K) and dexamethasone (d), and is currently under investigation as a targeted therapy in relapsed/refractory (R/R) MM. Using next-generation sequencing (NGS) and 18F-Fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT), we aimed to comprehensively evaluate minimal residual disease (MRD) in R/R MM patients (pts) treated with VenKd.
Methods: In this phase 2, dose-escalation study (NCT02899052), R/R MM (1 - 3 prior lines of therapy and no prior K exposure) pts received VenKd: Ven 400 mg/day + K 27 mg/m2 d1,2,8,9,15,16 + d 40 mg d1,8,15,22 (Cohort 1), same regimen but with Ven 800 mg/day (Cohort 2), Ven 800 mg/day + K 70 mg/m2 d1,8,15 + d 40 mg d1,8,15, 22 (Cohort 3/expansion cohort), or Ven 800 mg + K 56 mg/m2 d1,2,8,9,15,16 + d 40 mg d1,2,8,9,15,16,22,23 (Cohort 4). The following biomarker analyses were performed by central laboratory assessments of CD138-enriched bone marrow mononuclear cells collected at baseline: BCL2 gene expression by quantitative PCR and cytogenetic abnormalities by interphase fluorescence in situ hybridization. MRD assessments by NGS (clonoSEQ®) were performed on bone marrow aspirates at cycle 3 day 1 in pts achieving VGPR or better, time of suspected CR/sCR, and 6- and 12-months post confirmation of CR/sCR with negativity determined at <10-5 threshold. FDG-PET/CT imaging was performed on a subset of pts at baseline, cycle 3 day 1, and confirmation of CR or sCR, which corresponded to the bone marrow MRD evaluations by NGS. Lesions assessed by PET/CT were guided by standard of care imaging (i.e., x-ray, CT), and FDG-uptake was measured by maximum standardized uptake value. Pts were excluded from subsequent FDG-PET imaging based on proximity of evaluable lesions to anticipated areas of high normal FDG uptake (e.g., brain), or PET negative based on baseline FDG-PET imaging. MRD negativity (NGS and/or Imaging) was evaluated in the ITT population and key biomarker-defined subgroups (t(11;14) and BCL2high). Pts with missing or indeterminate assessments were considered MRD positive. Correlation with PFS, DOR, OS, and patient-reported outcomes (e.g., physical functioning, pain scores, fatigue) will be presented.
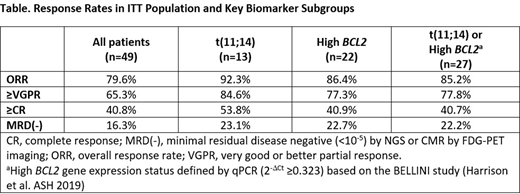
Results: As of 14 Feb 2020, 49 pts were enrolled (4 in cohort 1, 3 in cohort 2, 7 in cohort 4 and 35 in cohort 3 + expansion). Pts had received a median of 2 (1-3) prior lines of therapy, 96% were exposed to PI (57% refractory), 90% exposed to IMiD (71% refractory), and 86% exposed to PI + IMiD (45% double refractory). Median age was 60 years (37 - 79), 61% had ISS II/III disease, 27% had t(11;14), and 45% were BCL2high. Of note, 8 out of the 22 (36.4%) BCL2high pts were t(11;14) positive. Overall response rate (ORR) was 80% (≥PR), including 65% ≥ VGPR and 41% ≥CR (Table 1). Among t(11;14) pts, ORR was 92%, ≥ VGPR 85%, and ≥CR 54%; while among BCL2high pts ORR was 86%, ≥ VGPR 77%, and ≥CR 41%. Of the 19 pts assessed for MRD by NGS, 15 (79%) had clonotypes identified at baseline. Of these 15 pts, 6 (40%) achieved MRD negativity (<10-5) by NGS in the bone marrow after VenKd treatment. Of the 12 pts who participated in the FDG-PET sub-study, 10 (83%) were FDG-PET positive at baseline, and 8 (67%) completed post-treatment FDG-PET imaging. Of these 8 pts, 3 (38%) achieved complete metabolic response (CMR) by FDG-PET imaging after VenKd treatment. While only 4 pts were evaluated concurrently for MRD by NGS and FDG-PET/CT imaging, the assessments were concordant for 3 pts (2 positive, 1 negative). The discordant result (NGS negative, FDG-PET/CT positive lymph node) indicated clearance of disease in the bone marrow while the presence of a potential soft tissue plasmacytoma remaining after treatment with VenKd. Of the 19 pts evaluated by either NGS or FDG-PET/CT, 8 (42%) achieved MRD negativity by NGS in the bone marrow or CMR by FDG-PET/CT after VenKd treatment. The highest rates of MRD negativity were observed in t(11;14) and BCL2high subgroups (Table).
Conclusions: The combination of VenKd demonstrates promising efficacy in pts with R/R MM, including high rates of MRD, particularly in the t(11;14) and BCL2high subgroups. Overall, MRD assessments by NGS and FDG-PET/CT were highly concordant in this study and may be complementary for assessment of disease clearance in MM.
Costa:Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Genentech: Consultancy; BMS: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy; Celgene: Consultancy, Honoraria. Burwick:AbbVie: Research Funding. Jakubowiak:AbbVie, Amgen, BMS/Celgene, GSK, Janssen, Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive, Juno: Consultancy, Honoraria. Kaufman:Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Tecnopharma: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Karyopharm: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy; Celgene: Consultancy, Honoraria; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Sanofi/Genyzme: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Cabanillas:AbbVie: Research Funding. Dail:Genentech: Current Employment, Current equity holder in publicly-traded company. Karve:AbbVie: Current Employment, Current equity holder in publicly-traded company. Masud:AbbVie: Current Employment, Other: may hold stock or stock options . Yang:Abbvie: Current Employment, Current equity holder in publicly-traded company. Bueno:AbbVie: Current Employment, Current equity holder in publicly-traded company. Mudd:AbbVie: Current Employment, Current equity holder in publicly-traded company. Ross:AbbVie: Current Employment, Current equity holder in publicly-traded company. Davies:Oncopeptides: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene/BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotech: Honoraria; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal